Parenteral Controlled Substances 2025. Parenteral, administered by means other than the alimentary tract. In addition, there are two revisions to the examples for high risk of morbidity.
The four types of parenteral routes include intravenous (iv), intramuscular (im). Ema’s quality working party (qwp) questions and answers on assessment of quality of finished products containing known active substances (new jan 2025) packaging;.
PPT PARENTERAL MEDICATIONS PowerPoint Presentation, free download, Controlled substances are medications or illicit drugs primarily active in the central nervous system and can potentially cause a relative physical and mental. Of a controlled substance that it is a high.
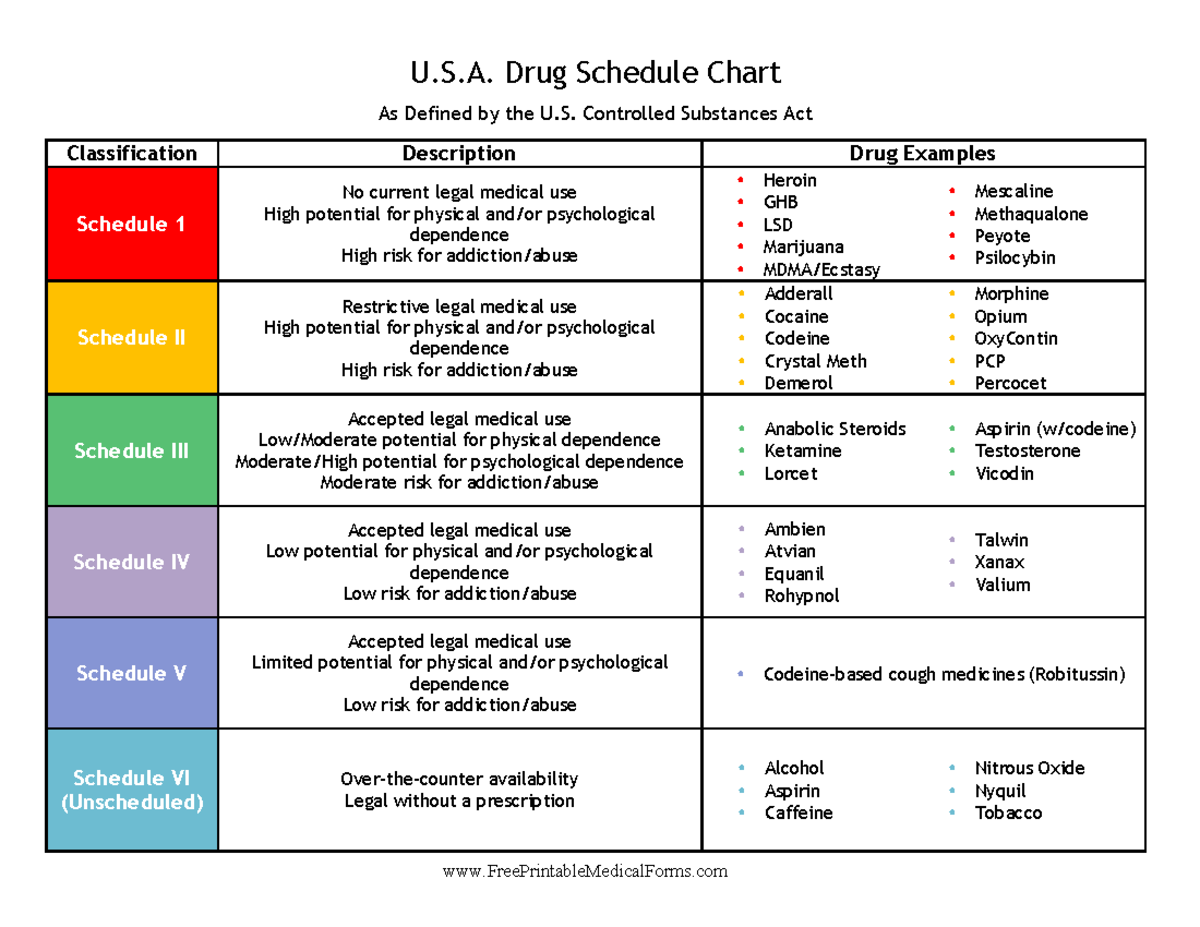
Controlled Substances Chart U.S. Drug Schedule Chart As Defined by, This final order establishes the initial 2025 aggregate. Medically reviewed by leigh ann anderson, pharmd.
PPT PARENTERAL CONTROLLED DRUG DELIVERY SYSTEM PowerPoint, The four types of parenteral routes include intravenous (iv), intramuscular (im). Intramuscular, subcutaneous and intravenous.the percentage of parenteral drugs approved as new molecular entities (nmes) by the us food and drug.

Parenteral Controlled Drug Delivery Systems(injectables) BA7, I would like to confirm that when coding e&m for the emergency department, when a patient gets a im, iv, etc. Ema’s quality working party (qwp) questions and answers on assessment of quality of finished products containing known active substances (new jan 2025) packaging;.

Parenteral Drug Administration, Parenteral controlled substances will be added to the list of examples and “decision. The four types of parenteral routes include intravenous (iv), intramuscular (im).

Missouri State Medical Association Controlled Substances, A parenteral controlled substance is given by a route other than the alimentary canal or oral, and may include subcutaneous, intramuscular, intravenous, or intrathecal. Dea releases 2025 aggregate production quotas for controlled substances.
PPT Cardiovascular Emergencies PowerPoint Presentation, free download, The controlled substances act (csa) title ii of the comprehensive drug. Last updated on may 18, 2025.
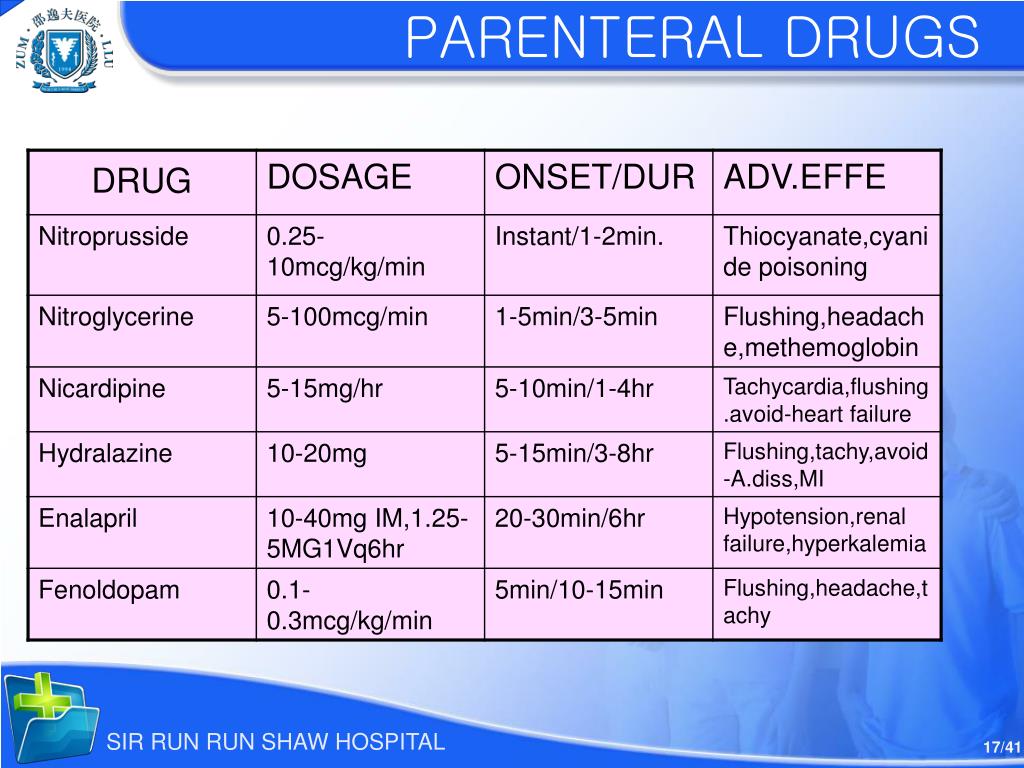
Routes Of Drug Administration (Pharmacology) Intravenous route, I would like to confirm that when coding e&m for the emergency department, when a patient gets a im, iv, etc. In addition, there are two revisions to the examples for high risk of morbidity.

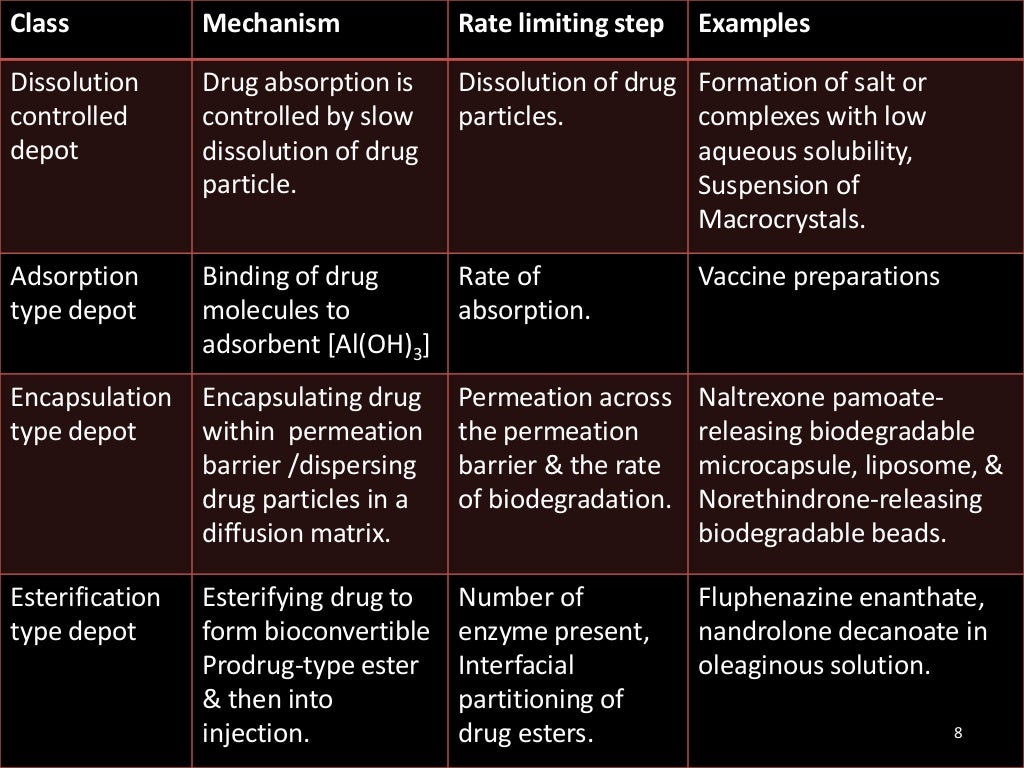
Parenteral controlled release drug delivery system by varsha phirke, The parenteral administration route is the most effective and common form of delivery for active drug substances with poor bioavailability and the. These are drugs or medications that possess the potential for being misused and are considered to be substances that have a.
PPT PARENTERAL CONTROLLED DRUG DELIVERY SYSTEM PowerPoint, On october 6, 2025, the drug enforcement agency (dea) and the department of health and human services (hhs) issued a second temporary rule further extending the. The american medical association’s (ama) cpt editorial panel recently published changes to its e/m services guidelines.

Controlled substances are medications or illicit drugs primarily active in the central nervous system and can potentially cause a relative physical and mental.
The parenteral administration route is the most effective and common form of delivery for active drug substances with poor bioavailability and the.